A Brief History of the Photoelectric Effect
In 1887 scientist Heinrich Hertz discovered the photoelectric effect while experimenting with a device called the spark gap generator which is a precursor to the radio. What Hertz found while using this device was “…sparks generated between two small metal spheres in a transmitter induce sparks that jump between between two different metal spheres in a receiver.” (https://physics.info/photoelectric/) The sparks that were jumping across the gap were, in fact electrons, which were receiving energy from ultraviolet light. In other words “when ultraviolet light shines on two metal electrodes with a voltage applied across them, the light changes the voltage at which sparking takes place.” (https://www.britannica.com/science/photoelectric-effect) This was an important finding because up until this point people where unaware of the relationship between light and electricity.
JJ Thompson, who many remember for his “plum pudding” model of the atom, discovered that the particles that were freed in the photoelectric effect were the same particles observed in the cathode rays he had been working with. His research using the cathode ray tube led to the discovery of corpuscles which we now know as electrons.

In 1902 Philipp Lenard made a shocking discovery. He found that as the frequency of light increased so to did the energy of the electrons. The expected result was that as the intensity or brightness of light increased the energy of the electron would increase. The experimental observation did not match the accepted theory of the time. So what does all this mean and who could make sense of it all? Before we get to that let’s discuss what specifically the photoelectric effect is.
What is the Photoelectric Effect?
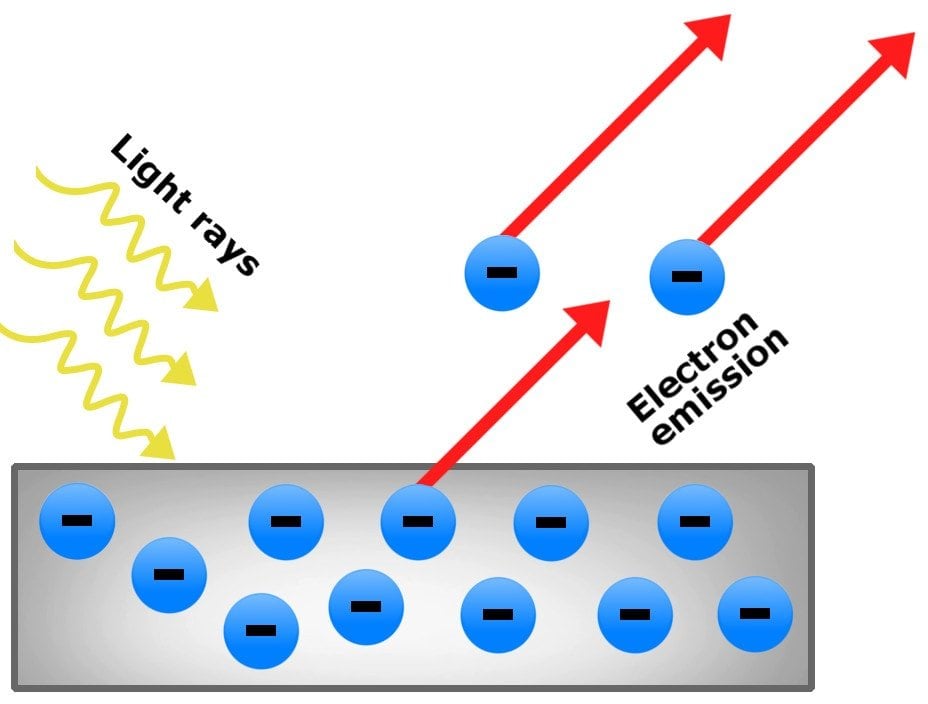
So we know a little about the history of the photoelectric effect and we know that it has something to do with electrons and the frequency of light. So what is it exactly? Great question, glad you asked. The photoelectric effect is a phenomenon which occurs when a light of a high enough frequency is shown onto a photo-sensitive metal resulting in the ejection of electrons from that metal. If the threshold frequency is not high enough then no electrons will be ejected. The threshold frequency varies for different metals and is the minimum frequency required for electrons to be ejected.
Increasing the intensity of the light will result in more electrons being ejected provide the frequency is at or above the threshold frequency. A high intensity light at a frequency below the threshold frequency will not result in the ejection of electrons, in other words the photoelectric effect will not be observed. Increasing the frequency of light results in an increase in kinetic energy of the photons. The intensity of light had no impact on the kinetic energy of the photons only on the number of photons being ejected.
Einstein and the Photoelectric Effect
Einstein is of course known for his theory of general and special relativity, his work with quantum mechanics, and his famous equation E=mc^2. What you might not realize is that Albert Einstein was awarded the 1921 Nobel Prize in physics for “for his services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect.” (https://www.nobelprize.org/prizes/physics/1921/summary/)
As you now know, Einstein was not the first to observe the photoelectric effect, so why is he so often credited and associated with it rather than Hertz, Lenard, or Thompson? He was the first to accurately describe how it occurs and to make the ground-breaking discovery relating waves and particles.
Einstein realized two facts regarding the photoelectric effect: 1) light is made of particles called photons and 2) the metal can only absorb the entire photon and not any other portion of it, think of it as an all or nothing proposition. Some of the energy of the photon that is absorbed is used to free the electron and the rest is converted to kinetic energy of the photon. The energy required to free the electron is called the work function. The strict definition of the work function is “energy (or work) required to withdraw an electron completely from a metal surface.” (https://school.eb.com/levels/high/article/electronic-work-function/32337)
What does all this mean? The results that Einstein observed were not in agreement with the accepted theory of the time. A new model of light was needed to match observation. Einstein had the insight to recognize that sometimes light acted as a wave and sometimes it acted as a particle. This was a stunning revelation that shocked the scientific community. Sir Isaac Newton thought light must be made of particles in order for it to experience reflection and refraction while Robert Hooke had argued that light had wave like behavior. Finally Einstein came along and settled the argument: light acts as both a wave and a particle.
Today we refer to this as the wave-particle duality or the dual nature of light. In general light travels as a wave and interacts with matter as particles called photons. Light is quantized meaning it is packaged in discrete units or particles which are called photons. In 1900 Max Planck, the father of quantum mechanics derived the equation for the energy of electromagnetic radiation, including light. Here is a short video describing Einstein and his contributions to the photoelectric effect: https://youtu.be/0b0axfyJ4oo

If we use the above equation and compare it to the known work function of a specific metal we can determine if the photoelectric effect will occur. The energy of the photon must be greater than the work function in order for the photoelectric effect to be observed. Interestingly enough, the electrons that were ejected from the metal end up falling back into the metal almost immediately.
What Did We Learn from the Photoelectric Effect?
We now know some of the history of the photoelectric effect and what the photoelectric effect is all about. Let’s take a moment and summarize what we learned from this scientifically significant discovery. Most importantly we leaned that classical physics can not accurately predict what happens at the atomic level. Classical physics predicted that increasing the intensity of light should increase the energy of the photons. What actually happened was that increasing the frequency of light resulted in an increase in the energy of a photon while increasing the intensity of light only resulted in an increases in the number of photons being ejected. Einstein was able to determine the energy of a photon by the equation E=hv and that the energy of the photon must be greater than the work function in order to be ejected from the metal. The photoelectric effected demonstrated the dual nature of light, that is it has both wave and particle behavior, in general light travels as a wave and interacts with matter as particles.